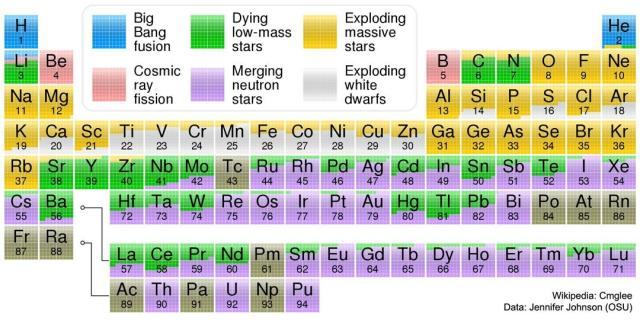
Where Your Elements Came From
Image Credit & License: Wikipedia: Cmglee; Data: Jennifer Johnson (OSU)
apod.nasa.gov/apod/ap230108.ht… #APOD
APOD: 2023 January 8 – Where Your Elements Came From
A different astronomy and space science related image is featured each day, along with a brief explanation.apod.nasa.gov
Andii אַנדִֽי
in reply to (moving) APOD • • •JamesTDG
in reply to (moving) APOD • • •Warwick Sanderson
in reply to (moving) APOD • • •Maisie Summers
in reply to (moving) APOD • • •verb (printfJess) 🦄
in reply to (moving) APOD • • •Hmmm
in reply to (moving) APOD • • •sn 🐦⬛
in reply to (moving) APOD • • •NeoFox
in reply to (moving) APOD • • •Mackaj
in reply to (moving) APOD • • •My first thought was: how do elements created from merging neutron stars escape? I know they're insanely dense with gravity some 2 billion times stronger than the Earth.
So I googled it and discovered that material is still ejected in an event called a "kilonova". So named because its peak brightness is about 1000 times that of a typical nova.
It's impossible to imagine the energy involved in events like that.
#elements #nova #gravity #kilonova #neutronstars #periodictable
ya
in reply to (moving) APOD • • •Morgana
in reply to (moving) APOD • • •gerald
in reply to (moving) APOD • • •i guess:
Carl Sagan 👉 we all are made of stardust
i think this is even more exciting than any religious myth of creation
Carioca
in reply to (moving) APOD • • •Luke Collie
in reply to (moving) APOD • • •BagheeraAltered
in reply to (moving) APOD • • •Peter Keating
in reply to (moving) APOD • • •Martin Vermeer FCD
in reply to (moving) APOD • • •royalsocietypublishing.org/doi…
#Nucleogenesis #Nucleosynthesis #OriginOfTheElements
Karoline
in reply to (moving) APOD • • •Anton
in reply to (moving) APOD • • •Steve Oh 🇺🇸💙✊🟰🗽🇺🇦
in reply to (moving) APOD • • •Erryn Pollock
in reply to (moving) APOD • • •For those curious (like me), the original/more complete version is at:
en.wikipedia.org/wiki/Chemical…
TLDR; The undescribed "brown" is "Human synthesis" or "No stable isotopes"
species of atoms having the same number of protons in the atomic nucleus and the same chemical properties, but not nessarily the same mass, or the same stability (or half-lifetime if they are unstable)
Contributors to Wikimedia projects (Wikimedia Foundation, Inc.)Monica Ayhens-Madon
in reply to (moving) APOD • • •Exhibitions - Planetarium (EN)
Planetarium (EN)Peter Widmayer (MOVED)
in reply to (moving) APOD • • •Barky
in reply to (moving) APOD • • •Daburu Dar
in reply to (moving) APOD • • •Hiding in Books
in reply to (moving) APOD • • •Doopdeedoop
in reply to (moving) APOD • • •Doc Edward Morbius ⭕
in reply to (moving) APOD • • •What's interesting / ironic is that helium on Earth is almost all ... of neutron-star origin.
That is, it's formed within the Earth's crust and core through decay of radioactive elements --- heavy stuff such as uranium, thorium, and plutonium naturally occurring. These give off beta particles --- a pair of protons and neutrons, which capture electrons and emerge as helium gas, a/k/a helium-4. Most of what humans capture is trapped in natural gas deposits and is recovered as part of the processing of gas wells.
So we get the second-most-abundant element in the Universe which is normally created from the Big Bang directly or stellar fusion through the long round-trip of neutron-star collisions and geological processes.
MrsMurilloToYou
in reply to (moving) APOD • • •Pat Squall Hey
in reply to (moving) APOD • • •Eileen60
in reply to (moving) APOD • • •